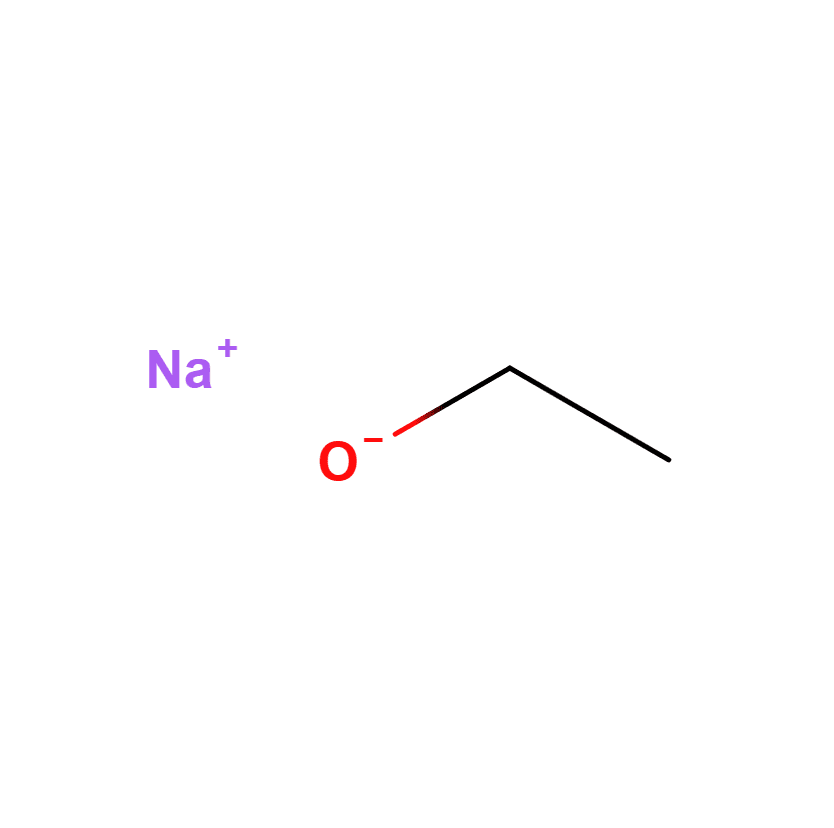
Sodium Ethoxide Solution
CAS : 141-52-6
Sodium Ethoxide Solution
Sodium ethoxide is known for its strong basicity, making it a potent nucleophile. Sodium ethoxide is a versatile compound with significant applications in organic synthesis across various industries. Its strong basic properties and reactivity make it indispensable in the production of pharmaceuticals, agrochemicals, aroma chemicals, fine chemicals, Dyes and polymers. By enabling precise control over chemical reactions, sodium ethoxide plays a vital role in advancing industrial processes and research in organic chemistry.
Types of Reactions
1. Condensation Reactions:
Sodium ethoxide participates in condensation reactions where it facilitates the formation of carbon-carbon or carbon-oxygen bonds. One prominent example is the Claisen condensation, where sodium ethoxide deprotonates esters or other carbonyl compounds to generate enolates, which then undergo condensation with another carbonyl compound, leading to β-keto esters or β-diketones.
2. Esterification:
As a strong base, sodium ethoxide is crucial in esterification reactions. It reacts with carboxylic acids or acid chlorides and alcohols to form esters and sodium salts. This process is essential in the synthesis of various organic compounds and is widely used in pharmaceutical and chemical industries.
3. Alkoxylation:
Sodium ethoxide facilitates alkoxylation reactions where it reacts with alkyl halides or alkyl sulfonates to introduce alkoxy (RO⁻) groups onto organic molecules. This reaction is important in the modification of organic compounds to improve their solubility or reactivity.
4. Etherification:
In etherification reactions, sodium ethoxide reacts with alkyl halides or alkyl sulfonates to form ethers. This process is significant in organic synthesis for the creation of ether linkages in complex molecules.
Industrial Applications of Sodium Ethoxide:
1. Pharmaceutical Industry:
Sodium ethoxide is utilized in pharmaceutical synthesis for the production of drugs and pharmaceutical intermediates. It plays a crucial role in the creation of complex organic molecules required for medications.
2. Chemical Synthesis:
In chemical industries, sodium ethoxide is a valuable reagent for organic synthesis processes. It facilitates the synthesis of a wide range of organic compounds, including fragrances, dyes, and fine chemicals.
3. Polymer Chemistry:
In polymerization reactions, sodium ethoxide can act as an initiator or catalyst for the formation of polymers, contributing to the production of plastics, resins, and other polymer-based materials.
4. Laboratory Research:
Sodium ethoxide is commonly used in organic chemistry laboratories for experimental purposes, where precise control over reactions and the synthesis of specific organic compounds is essential.
Physical and Chemical Properties
CAS Number | 141-52-6 |
Formula | C2H5ONa |
Appearance | Yellow to Dark Brown Liquid |
Assay | 19.0% to 21% w/w |
Free Alkalinity (NaOH + Na2CO3) | NMT 1.5% w/w |
Grade | Technical |
Packaging | 160kg HDPE Drums |
Why Choose Our Sodium Ethoxide Solution?
Our sodium ethoxide solution is manufactured under strict quality control standards to ensure consistent performance and reliable results. We are confident that our product will meet or exceed your expectations.
Subsribe To Our Email List
Stay in touch with us to get latest Product updates